
| Add: | 10th floor,Yatai Building,No.1,Shuyuanxi Rd,ChengDu 610016,China . |
| Tel: | 400-838-1985 028-84996634 |
| Fax: | 028-84971985 |
Validation Testing
The company has a professional testing engineer team and a full set of testing instruments, devoting to provide testing services with the FDA and EU-GMP standard for pharmaceutical companies.
The company has 20 professional engineers for validation and 10 full-time testing engineers. Most testing instruments and equipments were purchased from European or American brands, which are high precision, accuracy. We have the perfect management system, the complete personnel qualification certificate, equipment calibration certificate , standard operation procedures and all instruments are calibrated regularly.
Validation Modle
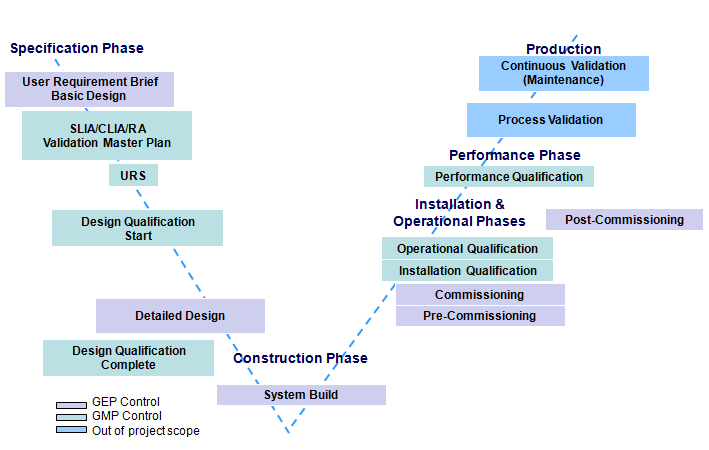
Validation Testing Services
▶ HVAC and cleanroom system:
HVAC, clean room, transfer box, laminar flow hood, biological safety cabinet, clean bench etc.
Main test items:
HEPA leakage testing, air flow pattern test, airflow test, Airborne particles count test, air change rate test,
differential pressure testing,recovery time testing,temperature and relative humidity testing,
illumination testing,AHU testing etc.
▶ Clean piping system verification test:
Lean piping system consists of purified water, water for injection, clean steam, compressed air, nitrogen gas etc.
The corresponding test items could be classified as:
Gas airborne microbes test, clean gas dew point test clean gas oil content test, clean gas Airborne particles
count test, pure steam quality test-non-condensable gas, dryness value, superheat value, pure steam
condensation water sampling.
▶ Pharmaceutical equipment qualification:
Including sterilization oven, all kinds of process equipment such as tanks, freeze-drying machine, rubber
stopper cleaning machine, filling/racking machine and so on, auxiliary equipment such as refrigerator,
incubator, stability test chamber, etc.
The main test items include:
The I/O test, clock accuracy test, noise test ,hepa DOP test, airborne particles count test, heat distribution
and heat penetration test, endotoxin experiment research, process temperature test, online sterilization
system test etc.
Process Validation
According to the effect of process parameters on the quality of the products, performing risk assessment to determine the risk level and key process parameters and verifying the key process parameters.
Continuous production process validation will be at least 3 batches of successful products, to prove the reliability of the technological process and reproducibility. Validation batch number should be able to ensure that the needs of the confidence interval of statistics. Samples have to be taken high frequency as far as possible in order to obtain enough information support to verify the conclusion.
Operators describe the required work sufficiently, according to production procedure, production process procedures.
Confirming listed key process parameters in the process of process validation.
According to the technological process and product quality standard to determine the sampling plan, reasonable arrangement of personnel for production samples of the products.
After the production process, finished product inspection should be done , product inspection results have to comply with the finished product quality standards, the statistical results should be recorded in the test data in the table.
According to testing results of the validation, summarize the steps of the process validation results should be summarized.
Tel:400-838-1985、028-84996634 Fax:028-84971985
Add:10th floor,Yatai Building,No.1,Shuyuanxi Rd,ChengDu 610016,China .
CopyRight © 2015 Cjtesting.com All rights reserved. Powered by: Juhewei.com